Most chemical eye injuries occur at work. Industries use a variety of chemicals daily, many of which are acidic or alkaline and have the potential to be corrosive to eye tissue.
The severity of a burn usually depends on the following three factors:
- What type of substance caused it – chemicals with a high and low pH (acids and alkalis) have the potential to cause greater damage.
- How long the substance had contact with the eye – the sooner the eye can be thoroughly rinsed for at least 15 to 30 minutes the better.
- How the injury is treated – the sooner the flushing begins makes a significant difference as damage can begin to occur within 3-5 seconds. Also important is the length of time the eye is flushed. Fifteen minutes is recommended in most cases, but alkali burns should be flushed for longer, usually 30 minutes or more. Finally, some saline eyewashes are available with a buffer solution that help neutralise the chemical splash. For example, Tobin’s Buffered Eyewash solution helps bring either an acid or alkali solution back to a neutral pH.
What happens when an acid or alkali solution gets in your eyes?
ACID BURNS
These burns are generally less destructive than their alkali counterparts and usually occur with exposure to strong acids that have a pH of less than 4. Hydrochloric acid (used to clean swimming pools) and sulfuric acid (found in car batteries) are some of the more common acids encountered in emergency settings. Acids tend to denature, coagulate, and precipitate corneal proteins on contact, creating a barrier that prevents deeper penetration of the acid. This protein coagulation produces the ground-glass appearance of the cornea often seen in severe acid burns. An exception to this is hydrofluoric acid (used in anti-rust solutions and glass etching). The fluoride ion rapidly penetrates the entire thickness of the cornea through cell membranes, causing significant corneal and anterior segment destruction. A buffer solution should always be used when working with hydrofluoric acid to rapidly neutralise the solution.
ALKALI BURNS
Chemicals commonly responsible for alkali injuries of the eye include sodium hydroxide (lye; found in drain cleaners and industrial cleaning solutions), ammonia (found in household cleaning solutions and fertilizers), and calcium hydroxide (lime; found in cement and plaster).
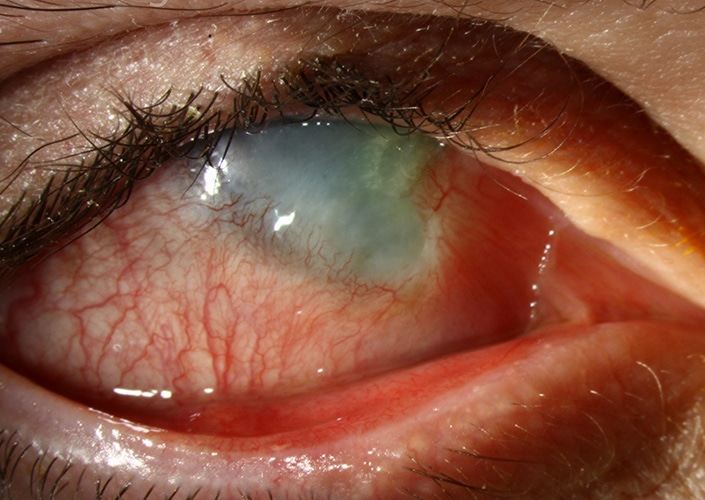
Alkali chemicals are lipophilic and penetrate cell membranes. Hydroxyl ions, which are common to many alkali chemicals, denature the collagen matrix of the cornea and facilitate further chemical penetration. Affected tissues can undergo liquefactive necrosis, in which the inflammatory response triggers release of proteolytic enzymes, leading to a cascade of damage. Potent alkalis can reach the anterior chamber in less than 15 seconds, causing destruction of tissues in the cornea and anterior chamber (including the trabecular meshwork, lens, and ciliary body). Penetration can continue to occur long after the initial exposure takes place. (Source: American Academy of Ophthalmology)
Eye damage can happen rapidly and can be life changing if not treated immediately. Permanently plumbed emergency eyewashes are ideal for industries that operate in a fixed locations with access to mains water supply. Make sure to select a manufacturer that meets the Australian and New Zealand Standard (AS4775) or the US Standard (ANSI Z358). For more details of what the standard requires you can download our compliance guide here.

Saline based eyewashes are ideal as a supplement to plumbed eyewashes or when mains water supply is not available, for example when working remotely, or on transport vehicles such as concrete trucks. Saline also has the benefit of needing no maintenance and being 100% sterile. For a full range of saline eyewashes click here.
For more information about your requirements and or to talk through how we can help, contact us on 0800 323223, enquiries@dilnz.co.nz or via the website.







